Difference Between Half Equivalence Point And Equivalence Point . if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point is the point. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a.
from www.chegg.com
The equivalence point is the point. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a.
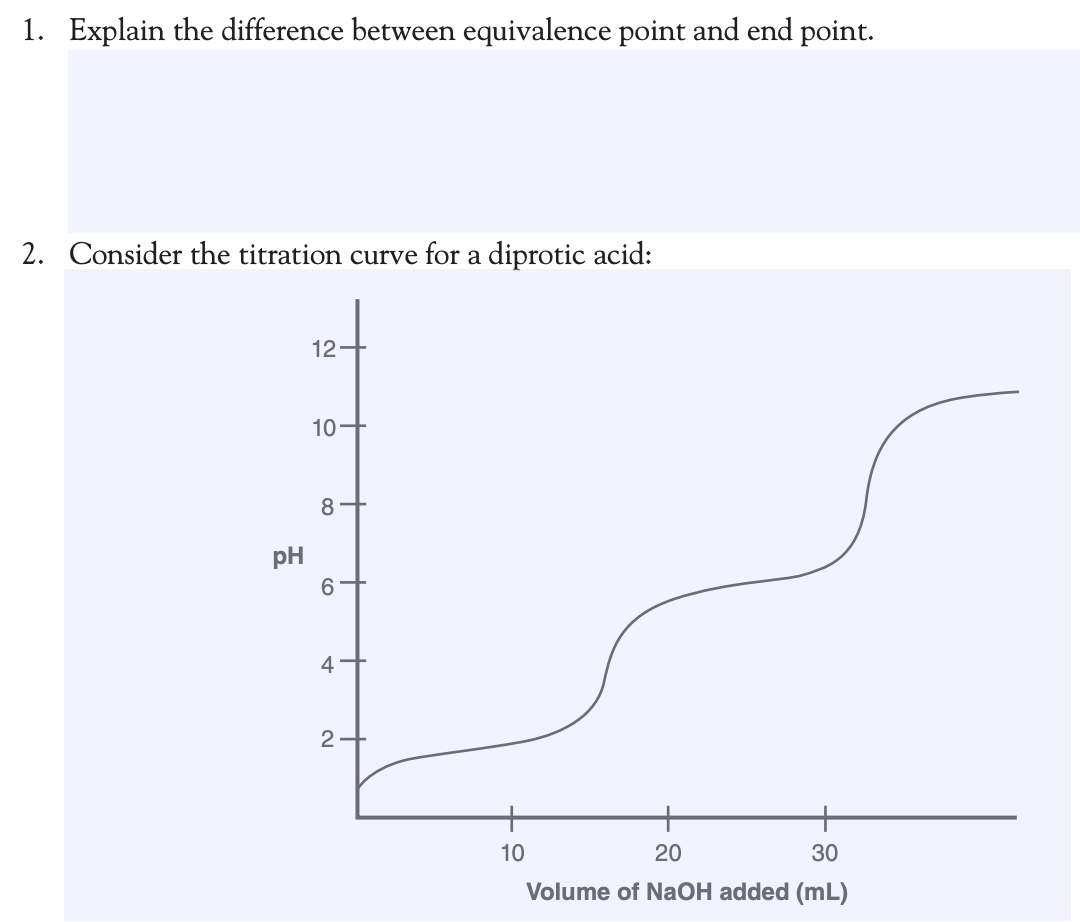
Solved 1. Explain the difference between equivalence point
Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically.
From erikfersherman.blogspot.com
How to Calculate Ph at Half Equivalence Point Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. if you are titrating an acid against a base, the half equivalence point will be. Difference Between Half Equivalence Point And Equivalence Point.
From askanydifference.com
Endpoint vs Equivalence Point Difference and Comparison Difference Between Half Equivalence Point And Equivalence Point The equivalence point is the point. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.differencebetween.net
Difference Between Endpoint and Equivalence Point Difference Between Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记8.1.1 Finding Ka Values翰林国际教育 Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point of a chemical reaction is the point. Difference Between Half Equivalence Point And Equivalence Point.
From marielanewslee.blogspot.com
Explain the Difference Between End Point and Equivalence Point Difference Between Half Equivalence Point And Equivalence Point The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition, Properties, Examples Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point is the point. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Difference Between Half Equivalence Point And Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. if you are titrating an acid against a base, the half equivalence point will be. Difference Between Half Equivalence Point And Equivalence Point.
From thecontentauthority.com
Equivalence Point vs Endpoint Meaning And Differences Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.pinterest.com
Equivalence Point Easy Science Chemistry, Action verbs, Chemical reactions Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free download ID570166 Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point. Difference Between Half Equivalence Point And Equivalence Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Difference Between Half Equivalence Point And Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point is the point. if you are titrating an acid against a base,. Difference Between Half Equivalence Point And Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point. pH and pKa relation Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is the point. if you are titrating an acid against a base,. Difference Between Half Equivalence Point And Equivalence Point.
From relationshipbetween.com
Difference Between Half Equivalence Point And Equivalence Point Relationship Between Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point. Difference Between Half Equivalence Point And Equivalence Point.
From the-equivalent.com
How to find half equivalence point The Equivalent Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. The equivalence point of a chemical reaction is the point. Difference Between Half Equivalence Point And Equivalence Point.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal in concentration. But Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point is the point. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From dxomjwenv.blob.core.windows.net
Titration Half Equivalence Point at Karen Winkleman blog Difference Between Half Equivalence Point And Equivalence Point The equivalence point is the point. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. if you are titrating an acid against a base,. Difference Between Half Equivalence Point And Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Difference Between Half Equivalence Point And Equivalence Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point is the point. The equivalence point of. Difference Between Half Equivalence Point And Equivalence Point.
From www.chegg.com
Solved 1. Explain the difference between equivalence point Difference Between Half Equivalence Point And Equivalence Point sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. if you are titrating an acid against a base, the half equivalence point will be. Difference Between Half Equivalence Point And Equivalence Point.